The Ozone Hole: From Discovery to Recovery
- Christian Poole
- Oct 12, 2023
- 8 min read
Updated: Dec 15, 2023
Lessons for the Green House Gas Issue

What Happened to the Ozone Hole?
The ozone hole was a major environmental concern in the 1980s and 1990s, with constant reports highlighting the devastating impact it could have on our planet. However, in recent years, the topic has not been in the spotlight as much. So, what exactly happened? While it may seem like the problem has gone away, the reality is that it is still very much present. It took decades of effort and phasing out of the chemicals responsible for ozone depletion to begin to have an effect.
In this article, we will explore the history of the ozone hole, what caused it, the efforts to address it, and what we can learn from this ongoing issue. The lessons learned show us, first of all, that combined global effort can have a positive effect if we act decisively. Secondly, as we will see, recovery is a slow process – the longer we wait, the more difficult it will be for the earth to recover from emissions. The greenhouse gas emissions from today will persist for decades to come. Limiting emissions while minimizing the impact on people’s needs must be a priority for humanity if we are serious about addressing the greenhouse gas issue.
What is Ozone?
Ozone is a naturally occurring molecule composed of three oxygen atoms (O3). It is formed through a complex series of chemical reactions involving oxygen molecules, sunlight, and other atmospheric components. In the Earth's atmosphere, ozone is found in the stratosphere and the troposphere.

In the stratosphere, which is located roughly 10-50 km above the Earth's surface, ozone forms the ozone layer and this is crucial for protecting life on Earth from harmful ultraviolet (UV) radiation. When UV radiation from the sun enters the atmosphere, it reacts with oxygen molecules to form ozone. The ozone layer acts as a shield, absorbing much of the harmful UV radiation before it can reach the Earth's surface.
In the troposphere, which is the lowest layer of the Earth's atmosphere and extends from the surface up to about 10-15 km, ozone is considered a pollutant. It is a major component of smog, which can have significant negative impacts on human health and the environment.
While ozone is essential in the stratosphere for protecting life on Earth from harmful UV radiation, it is important to limit its concentration in the troposphere to ensure the health of humans, animals, and plants.
Ozone in the Stratosphere
The stratosphere is unique in that it contains the majority of the Earth's ozone, which is essential for protecting life on our planet. As previously mentioned, ozone acts as a shield that prevents a lot of UV radiation that can cause a range of health problems for both humans and wildlife, including skin cancer, cataracts, and damage to DNA.

Interestingly, the stratosphere has its own climate system, which is distinct from the climate in the troposphere. The stratosphere's temperature actually increases with height, and this is the opposite of what happens in the troposphere. This is due to the presence of ozone, which absorbs the sun's energy and warms the surrounding air.
Despite its importance, the stratosphere is not immune to human impacts. The release of certain chemicals, such as chlorofluorocarbons (CFCs), can cause damage to the ozone layer by breaking down ozone molecules. This is why the international community came together in the 1980s to address the issue through the Montreal Protocol, which aimed to phase out the use of ozone-depleting substances. Today, we continue to monitor the health of the ozone layer and take action to protect it for future generations.
The Troposphere
Although ozone is an important component of our atmosphere, not all ozone is created equal. In fact, roughly 90% of the Earth's ozone is found in the stratosphere, where it protects us from harmful UV radiation. However, the remaining 10% of ozone is located in the lower part of the atmosphere, known as the troposphere, and this is the type of ozone we want to reduce.

Tropospheric ozone -also known as ground level ozone -can be caused by a variety of factors, including human emissions and hot weather. During heatwaves, we often hear of air quality advisories, which are issued when tropospheric ozone levels are elevated. This increase in ozone is generally not good for the health of many living things, including humans, and so we do our best to reduce it. By reducing our emissions and taking steps to improve air quality, we can help to keep tropospheric ozone levels in check and protect the health of our planet and its inhabitants.
Winners of the Nobel Prize
The discovery of the harmful effects of CFCs on the ozone layer was groundbreaking, and it had far-reaching implications for the entire planet. In fact, it was so important that it earned the scientists responsible for the discovery the Nobel Prize in Chemistry in 1995. This was a significant honor, as the Nobel Prize is widely regarded as one of the most prestigious awards in the world, recognizing those who have made outstanding contributions in their field.

The scientists who won the Nobel Prize for their work on the ozone layer were Mario J. Molina, F. Sherwood Rowland, and Paul J. Crutzen. Their discovery had a profound impact on the world, leading to a global effort to phase out the use of CFCs and other harmful chemicals that were contributing to the destruction of the ozone layer. This effort was successful, and the Montreal Protocol was signed in 1987, which committed nations to reduce their use of ozone-depleting substances. The original publication by Molina and Rowland can be found here. https://www.nature.com/articles/249810a0.
The Nobel Prize awarded to Molina, Rowland, and Crutzen was a recognition of the immense importance of their work and the impact that it had on the world. Their research not only helped to save the ozone layer but also served as a powerful reminder of the need to be vigilant about the impact of human activity on the environment.
The Montreal Protocol
The Montreal Protocol was a result of years of scientific research and advocacy by environmental groups, as well as growing public concern about the potential health and environmental impacts of ozone depleting substances (ODS). The agreement was initially signed by just 24 countries but eventually grew to be ratified by almost every country in the world. It set out a series of specific targets and timelines for phasing out the production and use of major ODS, including chlorofluorocarbons (CFCs), which were widely used in refrigeration, air conditioning, and aerosol products.
The success of the Montreal Protocol can be attributed to several factors:
Including the availability of viable alternatives to ozone-depleting substances (ODS)
The willingness of governments to cooperate and take action
The recognition of the potential economic and social benefits of reducing ODS
As a result of the agreement, levels of ODS in the atmosphere have been declining, and there are indications that the ozone layer is slowly recovering. The significance of the Montreal Protocol cannot be overstated, as it represents a rare example of international cooperation and action to address a global environmental problem.
After several rounds of negotiations and revisions, the Montreal Protocol was finally ratified by most countries in 2009. Its success is a testament to the power of international collaboration and the commitment to protecting our planet for future generations. The phase-out of ODS has resulted in a significant decrease in the levels of these substances serves as a model for addressing other environmental concerns through cooperative efforts.
Timeline of Events
It’s important to take a look at the detailed timeline of events surrounding the topic.
1974: Scientists F. Sherwood Rowland and Mario J. Molina propose a theory that man-made chlorofluorocarbons (CFCs) could have a lifetime of 40-150 years and potentially damage the stratospheric ozone layer, which protects living organisms on Earth from harmful UV radiation. However, this theory met with some initial skepticism.
1978: Several countries, including the United States, Canada, Norway, and Sweden, begin banning the use of CFCs in aerosol cans due to concerns about their potential impact on the ozone layer. However, the production of CFCs for other purposes continues to increase, leading to ongoing debates on the issue.
1983: British scientist Joseph Farman observes that the stratosphere above Antarctica had been dropping by roughly 35 percent, sparking concerns about the potential formation of an "ozone hole."
1985: Measurements of ozone levels over Antarctica become more frequent, and researchers begin to refer to the area with low ozone levels as the "ozone hole."
1987: The Montreal Protocol is created, with the aim of phasing out the production of several substances referred to as "ozone-depleting substances" (ODS), including CFCs. Many countries sign the agreement, representing a significant global effort to address the issue.
1995: Molina, Rowland, and Crutzen are awarded the Nobel Prize in Chemistry for their work on the ozone layer, including the discovery of the potential impacts of CFCs.
2000: The size of the ozone hole over Antarctica stops growing every year, indicating that the efforts to phase out ODS are having a positive impact.
2009: All United Nations member states ratify the Montreal Protocol, demonstrating global support for continued efforts to address the ozone depletion issue.
2022: The size of the ozone hole has only marginally changed since 2000, indicating that continued efforts to phase out ODS are necessary to maintain progress in protecting the ozone layer.
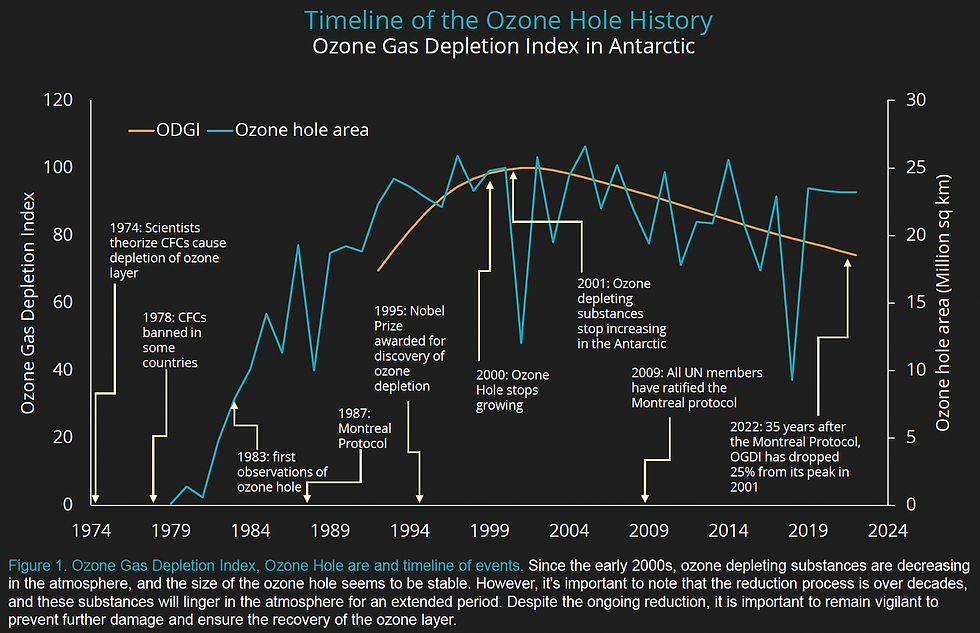
The Ozone Hole
While the adoption of the Montreal Protocol in 1987 was a pivotal moment in global efforts to protect the ozone layer, it took several decades to slow down the depletion of ozone in the stratosphere. As a result, the ozone hole has persisted to this day. Even though the size of the hole peaked in 2000, it has not shown any significant reduction since then. On the bright side, it has remained relatively stable without any increase in size.

Scientists continue to closely monitor the hole's size and composition, as well as the effectiveness of the policy changes, to ensure that the ozone layer is adequately protected. While it may be frustrating that it has taken so long to see progress, it is still a testament to the importance of sustained global efforts to address environmental issues.
Final Thoughts
We Must Take Action
The history of the depletion and recovery of the ozone layer shows that early action can make a significant difference in protecting the environment. By reducing the production of harmful substances like CFCs, we have been able to stabilize the ozone hole and start the process of healing the damage we caused. This is a clear indication that when we take action to protect the environment, it can lead to positive outcomes.
However, we should not stop at stabilization and instead continue to make efforts to ensure that the ozone layer recovers fully. This can only happen through sustained and concerted action by governments, businesses, and individuals worldwide. Ultimately, the ozone layer serves as a reminder of the profound impact that human activity can have on the planet and the importance of taking action to protect it.
We Can Apply This to Climate Change
The success story of the ozone hole serves as an inspiration and a call to action for other pressing environmental concerns, such as climate change. It demonstrates that proactive measures, international collaboration, and strong policies can make a substantial difference in addressing global environmental issues. The ozone hole's trajectory encourages us to tackle the challenges of climate change with urgency, seeking innovative solutions and adopting sustainable practices that will protect our planet and secure a prosperous future.
We Must Remain Vigilant
The history of the depletion and recovery of the ozone layer shows us that the decisions and policies we make now can have far-reaching consequences that may take many years or even decades to see any real impact. The gradual phasing out of CFCs, for example, took many years to implement and even longer to see results. However, this should not discourage us from taking action today to address other pressing environmental issues, such as climate change.
The lessons learned from the ozone crisis can guide us in our efforts to take timely, effective, and long-term action. It is important to remember that our actions now will have a significant impact on the world we leave for future generations. Therefore, it is our responsibility to take action now to protect our planet and ensure a sustainable future.
References
Contributors
Researchers
Mauro Aiello, Ph.D.
Axel Doerwald
Lead Author
Quinn Springett
Lark Scientific Financial Support
Axel Doerwald
Graphics
Adri Poggetti
To download this article, please click below:


